Programmable therapies for instability-driven cancers
Each candidate uses the same sensing-and-logic architecture but deploys a different effector to determine the outcome.
.png)
Each candidate uses the same sensing-and-logic architecture but deploys a different effector to determine the outcome.
.png)
This modularity allows us to advance multiple candidates in parallel, each tuned to a specific therapeutic role within the same decision-making framework.
We are early, but the pace comes from the platform itself. Logic scales.
Progressing through early research
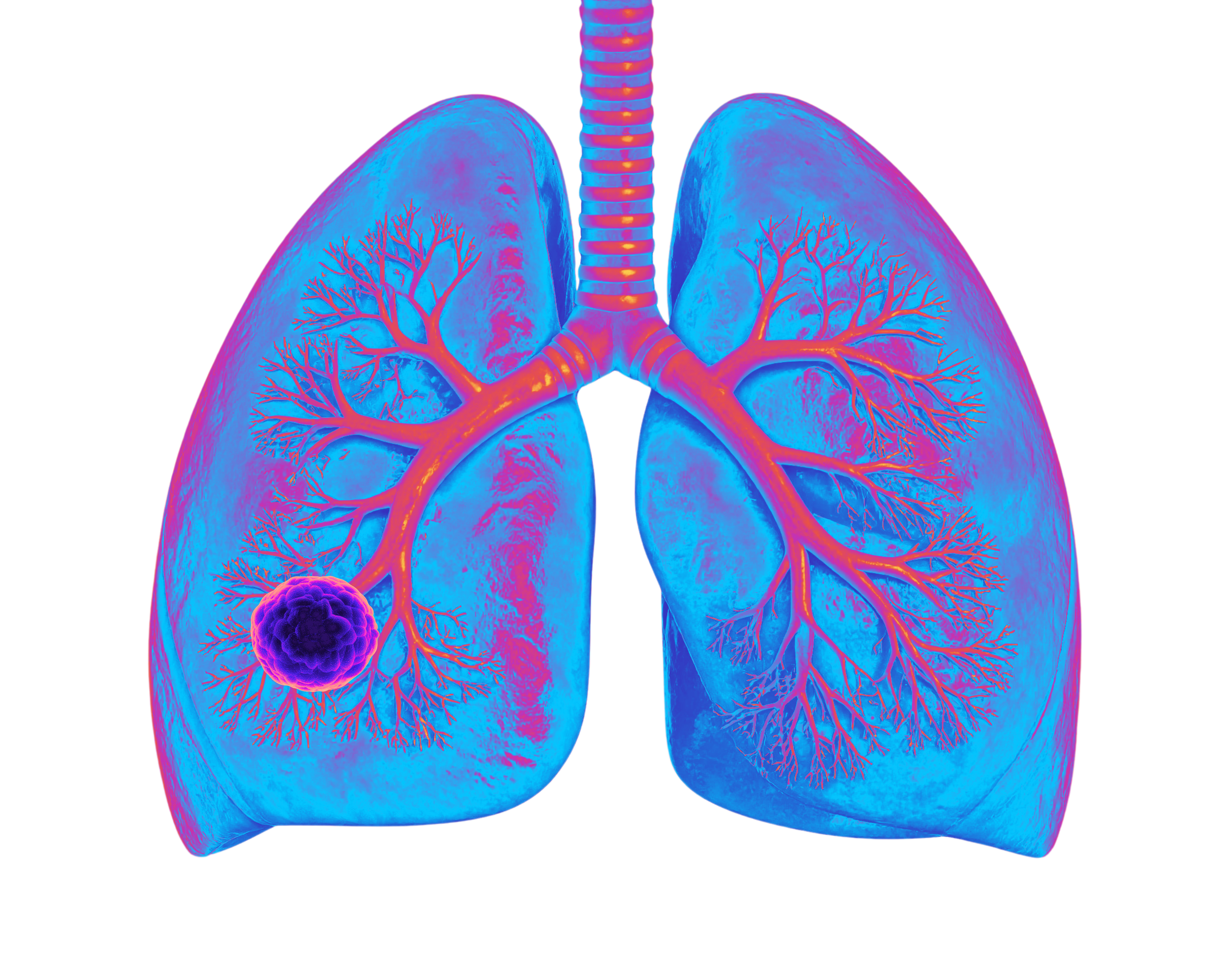
Where replication stress defines the disease
| Candidate | Mechanism | Indication | Discovery | Optimization | IND-Enabling |
|---|---|---|---|---|---|
| GenCTX-001 | CRISPR/Cas9 | SCLC | |||
| GenCTX-002 | Suicide gene | SCLC | |||
| GenCTX-003 | Prodrug-activated kill | SCLC | |||
| GenCTX-004 | Suicide gene | SCLC | |||
| GenCTX-005 | Prodrug-activated kill | SCLC |
Small cell lung cancer rapidly acquires resistance to platinum chemotherapy. As replication stress increases, tumors adapt, repair, or bypass pathways that treatments rely on. Relapse becomes almost inevitable.
GenCTX-001 uses our stress-responsive logic to activate CRISPR only in cells experiencing persistent genomic instability. Once activated, Cas9 disrupts genes associated with chemoresistance, opening a therapeutic window in which standard chemotherapy works again.
DTA is one of the most powerful suicide genes ever characterized - but impossible to use safely without precise control. Traditional promoter-based targeting leaks into healthy tissue, limiting its utility.
GenCTX-002 gives DTA strict, stress-gated activation. Only cells exhibiting sustained DNA-damage signals reach the ON state. The result is a high-potency effector deployed with logic-based precision.
Certain therapeutic strategies benefit from a dual-control system - one biological, one clinician-directed. Purely autonomous circuits may not always be optimal.
GenCTX-003 integrates our instability sensor with a drug-inducible caspase system, iCasp-9. The circuit ensures the effector is present only in the correct cells; clinicians determine when activation occurs.
Many tumors compromise mitochondrial (intrinsic) apoptosis. Caspase-8 bypasses this pathway entirely and initiates a clean extrinsic death signal.
GenCTX-004 activates caspase-8 selectively in cells under sustained stress. This enables a high-confidence death signal even when intrinsic pathways are disabled - a common feature of aggressive SCLC.
Prodrug systems offer excellent safety but require accurate targeting to avoid widespread toxicity. Traditional approaches cannot restrict activation tightly enough.
GenCTX-005 expresses HSV-Thymidine Kinase only in cells with persistent instability signals. When the prodrug is given, only these cells convert it into a lethal metabolite - concentrating effect where it's needed and nowhere else.

We are building therapies that let cells evaluate themselves and act accordingly - treatments that work where traditional approaches fail.
Learn how our sensing-and-logic architecture enables programmable therapeutics.
View Platform